Description
WHAT IS OXY IR 5MG?
Roxicodone 5 mg Anodyne (oxycodone hydrochloride tablets USP) is an opioid analgesic.
Each tablet for oral administration contains 5 mg, 15 mg or 30 mg of oxycodone hydrochloride USP.
Oxycodone hydrochloride is a white, odorless crystalline powder derived from the opiumalkaloid, thebaine. Oxycodone hydrochloride dissolves in water (1 g in 6 to 7 mL) and is considered slightly soluble in alcohol (octanol water partition coefficient is 0.7).
HOW ADDICTIVE CAN ROXICODONE 5 MG ANODYNE BE?
The 5 mg Roxicodone (oxycodone hydrochloride) tablet contains inactive ingredients: microcrystalline cellulose and stearic acid. The 15 and 30 mg tablets contain the following inactive ingredients: microcrystalline cellulose; sodium starch glycolate; corn starch; lactose; stearic acid; D&C Yellow No. 10 (15 mg tablet); and FD&C Blue No. 2 (15 mg and 30 mg tablets).
The 5 mg, 15 mg and 30 mg tablets contain the equivalent of 4.6 mg, 13.5 mg and 27.0 mg, respectively, of oxycodone free base.
WHAT IS IN AN OXYCODONE PILL?
INDICATIONS
ROXICODONE (oxycodone hydrochloride) ® tablets are an immediate-release oral formulation of oxycodone hydrochloride indicated for the management of moderate to severe pain where the use of an opioid analgesic is appropriate.
DOSAGE AND ADMINISTRATION
Roxicodone 5 mg Anodyne (oxycodone hydrochloride) ® is intended for the management of moderate to severe pain in patients who require treatment with an oral opioid analgesic. The dose should be individually adjusted according to severity of pain, patient response and patient size. If the pain increases in severity, if analgesia is not adequate, or if tolerance occurs, a gradual increase in dosage may be required.
Patients who have not been receiving opioid analgesics should be started on ROXICODONE (oxycodone hydrochloride) ® in a dosing range of 5 to 15 mg every 4 to 6 hours as needed for pain. The dose should be titrated based upon the individual patient’s response to their initial dose of ROXICODONE (oxycodone hydrochloride) ®. Patients with chronic pain should have their dosage given on an aroundthe-clock basis to prevent the reoccurrence of pain rather than treating the pain after it has occurred. This dose can then be adjusted to an acceptable level of analgesia taking into account side effects experienced by the patient.
For control of severe chronic pain, Roxicodone 5 mg Anodyne (oxycodone hydrochloride) ® should be administered on a regularly scheduled basis, every 4-6 hours, at the lowest dosage level that will achieve adequate analgesia.
As with any potent opioid, it is critical to adjust the dosing regimen for each patient individually, taking into account the patient’s prior analgesic treatment experience. Although it is not possible to list every condition that is important to the selection of the initial dose of ROXICODONE (oxycodone hydrochloride) ®, attention should be given to: 1) the daily dose, potency, and characteristics of a pure agonist or mixed agonist/antagonist the patient has been taking previously, 2) the reliability of the relative potency estimate to calculate the dose of oxycodone needed, 3) the degree of opioid tolerance, 4) the general condition and medical status of the patient, and 5) the balance between pain control and adverse experiences.
CONVERSION FROM FIXED-RATIO OPIOID/ACETAMINOPHEN, OPIOID/ASPIRIN, OR OPIOID/NONSTEROIDAL COMBINATION DRUGS
When converting patients from fixed ratio opioid/non-opioid drug regimens a decision should be made whether or not to continue the non-opioid analgesic. If a decision is made to discontinue the use of non-opioid analgesic, it may be necessary to titrate the dose of ROXICODONE (oxycodone hydrochloride) ® in response to the level of analgesia and adverse effects afforded by the dosing regimen. If the non-opioid regimen is continued as a separate single entity agent, the starting dose ROXICODONE (oxycodone hydrochloride) ® should be based upon the most recent dose of opioid as a baseline for further titration of oxycodone. Incremental increases should be gauged according to side effects to an acceptable level of analgesia.
PATIENTS CURRENTLY ON OPIOID THERAPY
If a patient has been receiving opioid-containing medications prior to taking ROXICODONE (oxycodone hydrochloride) ®, the potency of the prior opioid relative to oxycodone should be factored into the selection of the total daily dose (TDD) of oxycodone.
In converting patients from other opioids to ROXICODONE (oxycodone hydrochloride) ® close observation and adjustment of dosage based upon the patient’s response to ROXICODONE (oxycodone hydrochloride) ® is imperative. Administration of supplemental analgesia for breakthrough or incident pain and titration of the total daily dose of ROXICODONE (oxycodone hydrochloride) ® may be necessary, especially in patients who have disease states that are changing rapidly.
MAINTENANCE OF THERAPY
Continual re-evaluation of the patient receiving ROXICODONE (oxycodone hydrochloride) ® is important, with special attention to the maintenance of pain control and the relative incidence of side effects associated with therapy. If the level of pain increases, effort should be made to identify the source of increased pain, while adjusting the dose as described above to decrease the level of pain.
During chronic therapy, especially for non-cancer-related pain (or pain associated with other terminal illnesses), the continued need for the use of opioid analgesics should be re-assessed as appropriate.
CESSATION OF THERAPY
When a patient no longer requires therapy with Roxicodone 5 mg Anodyne (oxycodone hydrochloride) ® or other opioid analgesics for the treatment of their pain, it is important that therapy be gradually discontinued over time to prevent the development of an opioid abstinence syndrome (narcotic withdrawal). In general, therapy can be decreased by 25% to 50% per day with careful monitoring for signs and symptoms of withdrawal (see Drug Abuse and Dependence section for description of the signs and symptoms of withdrawal). If the patient develops these signs or symptoms, the dose should be raised to the previous level and titrated down more slowly, either by increasing the interval between decreases, decreasing the amount of change in dose, or both. It is not known at what dose of ROXICODONE (oxycodone hydrochloride) ® that treatment may be discontinued without risk of the opioid abstinence syndrome.
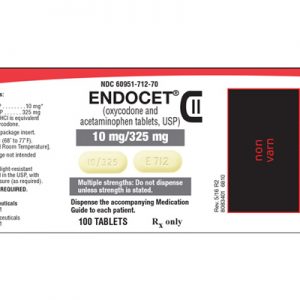
HOW SUPPLIED
ROXICODONE® (oxycodone hydrochloride tablets, USP) are available as follows:
5 mg white scored tablets (Identified 54 582)
[Embossed 54 582 on one side]
NDC 66479-580-25: Unit dose, 25 tablets per card, (reverse numbered), 4 card per shipper
NDC 66479-580-10: Bottles of 100 tablets
15 mg green tablets scored (Identified 54 710)
[Embossed 54 710 on one side]
NDC 66479-581-10: Bottles of 100 tablets
30 mg blue tablets scored (Identified 54 199)
[Embossed 54 199 on one side]
NDC 66479-582-10: Bottles of 100 tablets
DEA Order Form Required Dispense in a tight, light-resistant container. Protect from moisture.
Store at 25°C (77°F); excursions are permitted to 15°-30°C (59°-86°F) [see USP Controlled Room Temperature]