Description
CAN I BUY DEXEDRINE SPANSULE 5 MG DEXTROAMPHETAMINE SULFATE CAPSULES ONLINE
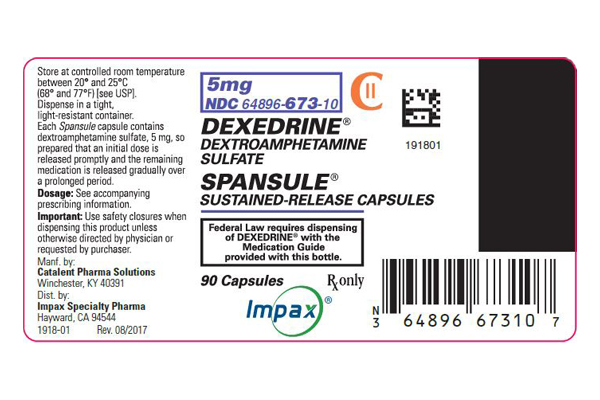
Dexedrine Spansule 5 mg Dextroamphetamine Sulfate is the dextro isomer of the compound d,1-amphetamine sulfate, a sympathomimetic amine of the amphetamine group. Chemically, dextroamphetamine is d-alpha-methylphenethylamine, and is present in all forms of DEXEDRINE as the neutral sulfate.
SPANSULE CAPSULES
Each SPANSULE sustained-release capsule is so prepared that an initial dose is released promptly and the remaining medication is released gradually over a prolonged period.
Each capsule, with brown cap and clear body, contains dextroamphetamine sulfate. The 5-mg capsule is imprinted 5 mg and 512 on the brown cap and is imprinted 5 mg and ap on the clear body. The 10-mg capsule is imprinted 10 mg-513-on the brown cap and is imprinted 10 mg-ap-on the clear body. The 15-mg capsule is imprinted 15 mg and 514 on the brown cap and is imprinted 15 mg and ap on the clear body. A narrow bar appears above and below 15 mg and 514. Product reformulation in 1996 has caused a minor change in the color of the time-released pellets within each capsule. Inactive ingredients now consist of cetyl alcohol, D&C Yellow No. 10, dibutyl sebacate, ethylcellulose, FD&C Blue No. 1, FD&C Blue No. 1 aluminum lake, FD&C Red No. 40, FD&C Yellow No. 6, gelatin, hypromellose, polyethylene glycol, povidone, sodium lauryl sulfate, sugar spheres, and trace amounts of other inactive ingredients.
NARCOLEPSY DEXEDRINE SPANSULE 5 MG DEXTROAMPHETAMINE SULFATE
ATTENTION DEFICIT DISORDER WITH HYPERACTIVITY
As an integral part of a total treatment program that typically includes other measures (psychological, educational, social) for patients (ages 6 years to 16 years) with this syndrome. A diagnosis of Attention Deficit Hyperactivity Disorder (ADHD; DSM-IV) implies the presence of the hyperactive-impulsive or inattentive symptoms that caused impairment and were present before age 7 years. The symptoms must cause clinically significant impairment, e.g., in social, academic, or occupational functioning, and be present in 2 or more settings, e.g., school (or work) and at home. The symptoms must not be better accounted for by another mental disorder. For the Inattentive Type, at least 6 of the following symptoms must have persisted for at least 6 months: lack of attention to details/careless mistakes; lack of sustained attention; poor listener; failure to follow through on tasks; poor organization; avoids tasks requiring sustained mental effort; loses things; easily distracted; forgetful. For the Hyperactive-Impulsive Type, at least 6 of the following symptoms must have persisted for at least 6 months: fidgeting/squirming; leaving seat; inappropriate running/climbing; difficulty with quiet activities; “on the go”; excessive talking; blurting answers; can’t wait turn; intrusive. The Combined Type requires both inattentive and hyperactive-impulsive criteria to be met.